Chemical engineering expertise helps in fight against COVID-19
From hand sanitisers and nasal sprays to improved PPE, the University of Birmingham’s School of Chemical Engineering and Healthcare Technologies Institute (based here in the ITM) have made significant contributions to the global response to the COVID-19 pandemic.

In March, staff set up a manufacturing facility at the Collaborative Teaching Laboratory to produce hand sanitiser, desperately needed at the time by frontline care workers to prevent the spread of COVID-19 due to a national shortage. The facility used chemicals donated from Schools across campus and produced 20 litre batches in accordance with the World Health Organization’s formulation for hand sanitiser. They dispensed the batches into 50ml containers, and packaged them for Birmingham City Council to distribute around the region.
Also amid our response has been a project led by Dr Sophie Cox, Dr David Bassett and Dr Thomas Mills, which saw students and staff from across campus team up to manufacture face visors for frontline medical staff.
The team used their 3D printing expertise to set up a production line at the Collaborative Teaching Laboratory, and used a design taken from a commercially tested blueprint that was supplied by Czech Republic-based PrusaPrinters. The 3D printing technology was used to produce the headband part of the visor, which fits across the user’s forehead, and is attached to a clear plastic visor.
The team produced 2,600 visors to provide additional protection for frontline workers treating people with COVID-19, and donated these to local care homes, hospices and hospitals.
From the outset of the pandemic, it was clear there were issues with wearing facemasks for prolonged periods, such as abrasion and bruising of facial tissues. By early April, Professor Liam Grover and Dr Sophie Cox had started a rapid and intensive collaboration with clinicians and research scientists from King’s College London, to improve the seal and fit of facemasks used by healthcare professionals.
The collaboration brought together expertise in facial imaging, skin interfacing devices and 3D printing, to produce a prototype for a customised mask seal that will improve comfort and reduce exposure risk for people who need to wear facemasks all day, every day.
The researchers worked with University of Birmingham Enterprise to file patent applications, and assign them to a company with an experienced management team and a network of manufacturing partners. MyMaskFit will use the technologies developed at Birmingham and King’s to deliver a service where the care worker scans their face using a smartphone, and within days receives a custom-made, reusable, medical grade facemask within days.

In the early stages of the pandemic, medical staff were faced with rising numbers of critically ill patients, and shortages of PPE. There was the very real concern about transmission during procedures such as intubation or CPR, which put pressure on the chest or forces exhalation of viral-laden aspirate. Dr Richard Williams, Research Fellow at the University of Birmingham’s Healthcare Technologies Institute, and Matthew Campbell-Hill, from the University’s Institute of Clinical Sciences, worked with clinicians and medical experts to come up with a solution. They designed a transparent pop-up tent that can be assembled in 20 seconds to cover the patient’s head and neck area and shield staff, and called their product AerosolShield. AerosolShield can be assembled in 20 seconds, is lightweight and collapsible for easy transport, storage and disposal, and has self-closing access points, allowing easy hand access in and around the patient’s airway, with full line of sight for medical staff.
The team went from design brief, to final design, securing a manufacturing deal and first orders in under a week. Since then AerosolShield devices have been used in GP practices and care homes around the UK, and helped protect an estimated 3,000 staff working in the frontline.
The science suggests that the COVID-19 virus is transmitted in aerosol droplets that not only carry, but also protect, the virus.
A team of researchers headed by Dr Zhenyu Jason Zhang is working on the development of additives that can be included in existing commercial products such as detergents, or integrated within packaging to form an invisible and long-lasting film of sub-micron thickness.
Recent evidence suggests that different surface characteristics such as porosity, rigidity, and roughness all affect the virus’ viability. The team will be drawing on their expertise in soft matter, surface chemistry, formulation engineering, microbiology and the product development capabilities offered by industrial partners to develop new antiviral sprays, films and other products.
The research will take place over the next 18 months in partnership with the University of Cambridge and three key industrial partners: Dupont Teijin Film, Innospec, and FiberLean with the aim of rapidly commercialising the formulations produced.
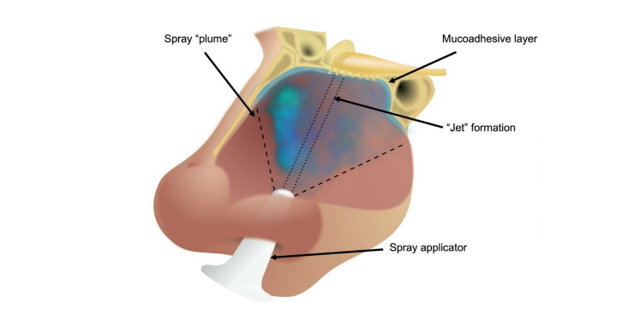
Researchers led by Professor Liam Grover and Dr Richard Moakes set out to create a formulation for an antiviral nasal spray that can be used as a prophylactic, and also to help prevent person-to-person transmission. Fully aware of the urgency of the situation, they developed a formulation from materials that are already approved by regulatory bodies in the UK, Europe and the US, widely used in medical devices, medicines or food products, and already manufactured to pharmaceutical grade.
They tested a range of compounds for their ability to ‘plume’ when sprayed using a typical nasal spray applicator, and to stay inside the nose after application, before selecting a gellan polysaccharide as the carrier for an antiviral agent, called λcarrageenan.
The researchers then tested the mixture at various different proportions for its antiviral affect in cell culture, and found it could deliver significant suppression of infection compared to untreated controls. This effect was seen up to 48 hours after application, and when the formulation was diluted many times.
University of Birmingham Enterprise filed a patent application for the formulation and is now seeking to license the patent to an organisation that is committed to manufacturing a consumer product and managing its distribution it to the widest possible audience.