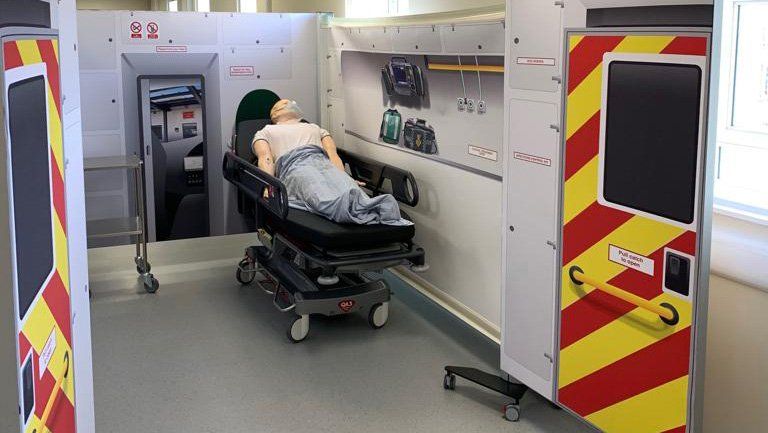
The Institute of Translational Medicine recently hosted the UK’s first demonstration of a remote-controlled ultrasound scan over a public 5G network, at an event which showcased how 5G can transform healthcare and the emergency services.
The demo, in partnership with BT and WM5G, was hosted by the Medical Devices Testing and Evaluation Centre’s (MD-TEC) prestigious simulation lab, located in the ITM. The showcase brings the concept of a 5G Connected Ambulance to life and provides new technologies to frontline staff, creating a facility for patients to be diagnosed and triaged in the most appropriate settings. It enables remote diagnostics to be performed by paramedics who are supported by clinicians back at the hospital.
This is a real-world example of how 5G will support digital transformation in the delivery of public services and enable care delivery to be streamlined. It is just one example of how activities which can only be performed in static environments today can become mobile tomorrow.
The demonstration simulates a paramedic in the field performing an ultrasound scan on a patient, under the remote guidance of a clinician who is able to interpret the ultrasound image in real-time. The ultrasound sensor is manipulated locally by the paramedic under the remote direction of the clinician, who uses a joystick to send control signals over the live 5G network to a robotic or ‘haptic’ glove worn by the paramedic. The glove creates small vibrations that direct the paramedic’s hand to where the clinician wants the ultrasound sensor to be moved, allowing the clinician to remotely control the sensor position, while seeing the ultrasound images in real time. In addition, there is a camera in the ambulance which transmits in high definition a view of the inside of the ambulance covering the patient and paramedic to a second screen located on the clinician’s workstation.
The images are relayed over a high-bandwidth 5G connection, so the clinician is able to view both the ultrasound examination performed by the paramedic and keep an eye on the overall scene inside the ambulance. The superfast speeds of 5G ensure sharper and more reliable imagery for the clinician than could previously be achieved.
Enabling ultrasound scans to be performed by paramedics in the field and reviewed remotely by an expert clinician should bring a number of advantages to patients and to the NHS. As well as speeding up diagnoses for patients, it has the potential to reduce the number of ambulance journeys and emergency department visits. This will improve the overall experience for patients while freeing up ambulance resources and reducing pressure on emergency departments. Faster diagnoses can also assist in triaging patients, ensuring more effective outcomes for the patient, and increasing overall efficiency for the hospital.
Tim Jones, Chief Innovation Officer at UHB, commented: “We are immensely excited about the potential of 5G to support transformation in healthcare. As a Global Digital Exemplar, we are always looking into new technologies and how we can use them to improve patient care. 5G will help us to roll out this next generation of healthcare technologies.
“Our clinicians will in the future be able to deliver holistic specialist advice in real time, potentially forming virtual multi-disciplinary teams to provide the best patient care using intelligent IT links. Information would be accessible at the point of need, ensuring informed decision making leading to improved patient safety, quality of care and patient/clinician experience.”
Dr Omkar Chana, Programme Director at WM5G said: “We are in a unique position where we are beginning to understand how developments in technology can be leveraged to change and improve the way in which some healthcare services are delivered. High definition clinical imaging is one example that lends itself well to digital transformation owing to the large amounts of data transferred in a short space of time.
WM5G’s role is to ensure there is a focus on delivering benefits that will have high levels of impact on the patient, the clinician and the hospital or organisation providing the service. This early demonstrator puts the technology in the hands of clinicians, and we want to work with them to continue to prove, test and scale this and other applications in the region.”
Gerry McQuade, CEO of BT’s Enterprise unit, said: “We’re really excited to be working with WM5G and University Hospitals Birmingham on the first 5G healthcare trial to take place in the UK over a live public network.
“BT has a long and proud heritage of working with the NHS to better connect patients and healthcare professionals and the characteristics of 5G will deliver a huge-step change in speed, capacity and reliability. We are focused on delivering new, innovative services which will make lives better and firmly believe in using the power of 5G to bring potentially life-saving benefits to patients. There’s no better place to start realising this vision than in Birmingham, part of the UK’s first multi-city 5G test bed.”
Ultrasonography is the second most common diagnostic test reported by the NHS, with more than 9.5 million carried out last year in England alone. On average, 408,000 patients attend UHB Emergency Departments each year, with 113,500 of these patients using an ambulance.
With BT’s EE mobile arm recently switching parts of Birmingham on to the UK’s first 5G services, the company is working with WM5G to illustrate how the technology can deliver significant benefits to the NHS and the wellbeing of citizens across the West Midlands region.